research Interest
Welcome to the Wohl lab!
Our major goal is to fight visual impairment and blindness at the cellular and molecular level. Many retinal diseases such as age-related macular degeneration (AMD), diabetic retinopathy glaucoma, or retinitis pigmentosa lead to impairment of vision and in most cases, blindness. Although the impairment of vision is often due to primary damage to the retinal neurons, glia cells play significant roles in this process and can induce secondary neuronal loss. We want to understand the role of glia during retinal diseases and our focus lies on Müller glia, the major glia cell type in the retina. We are interested in two major questions:
1) How can we regenerate a diseased retina?
2) How can we attenuate diseases and prevent additional damage to the retina?
Müller Glia
First a few words about Müller glia and why they are so important. Müller glia are fascinating cells. They are the predominant glia in the neural retina and are generated last during retinogenesis, the process when all six retinal neuronal cells and the Müller glia are formed. They are named after Professor Heinrich Müller who discovered and described them in 1851. These glia, similar to astrocytes in the brain, are the support cells in the retina and have a variety of other functions including maintaining the homeostasis of the tissue and protection after injury or disease. As part of their protective function, Müller glia undergo morphological and molecular changes to create a barrier to isolate damaged/diseased tissue from healthy tissue. This barrier however also creates a non-permissive environment for regeneration and is one of the reasons why our retinas cannot regenerate. Furthermore, this glial response, called gliosis, can trigger detrimental effects including secondary neuronal loss and inflammation. It is a very complex process and includes a variety of factors and mechanisms which are not fully understood.
But that’s not all. Müller glia can serve as an endogenous source for regeneration, at least in lower vertebrates such as fish. In the event of damage, fish Müller glia turn into stem-cell like cells, called retinal progenitor cells, divide a few times, and then give rise to new functional neurons that replace the lost ones. A remarkable capability that unfortunately is silenced in mammals including us humans. However, even our (mammalian) Müller glia have a molecular composition that is similar to retinal progenitor cells and many experts are eager to decipher the mechanisms of how to awaken Muller glia as an endogenous source for retinal regeneration in mammals. A way to force them to wake up is a process called reprogramming by artificially inducing factors that are naturally found in fish Müller glia during regeneration.
Molecules that play a role in both Müller glia-driven retinal regeneration in fish and gliosis in mammals are microRNAs.
microRNAs
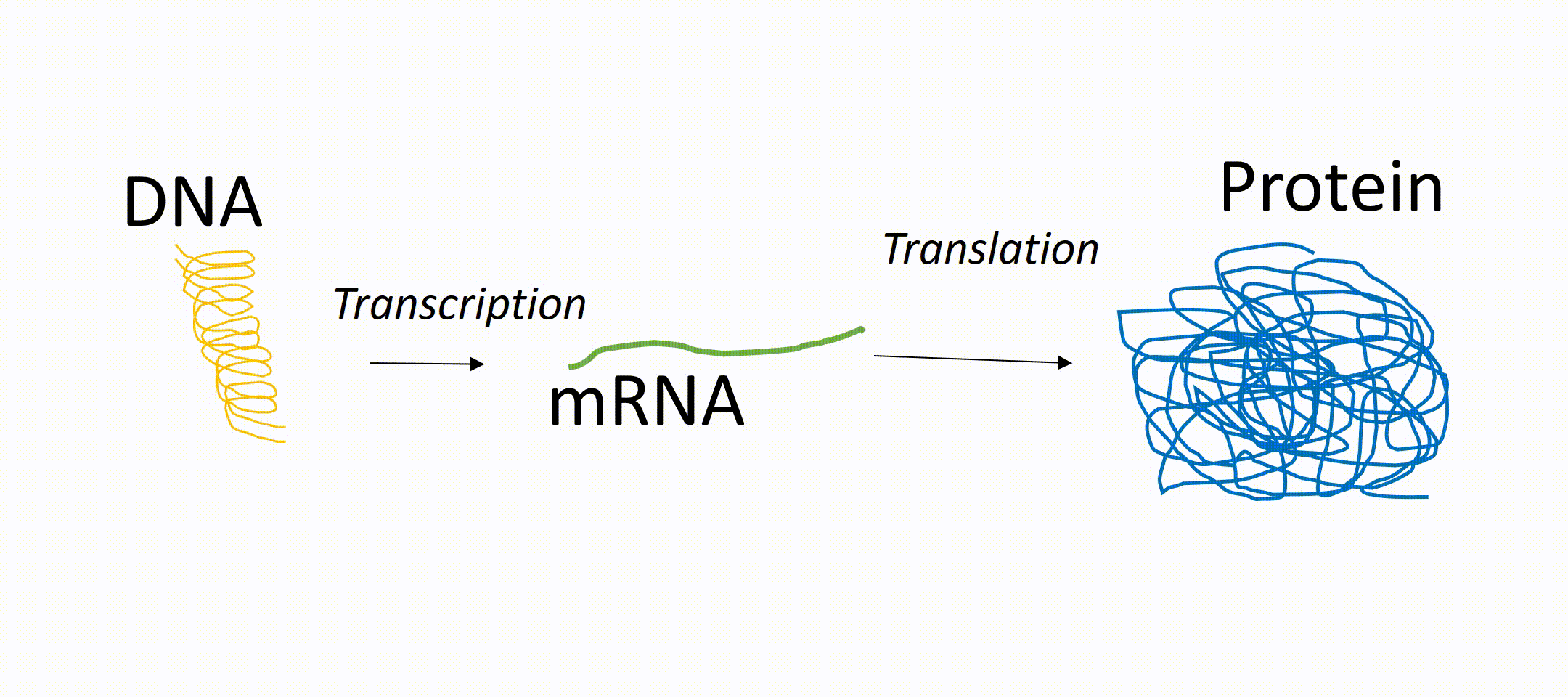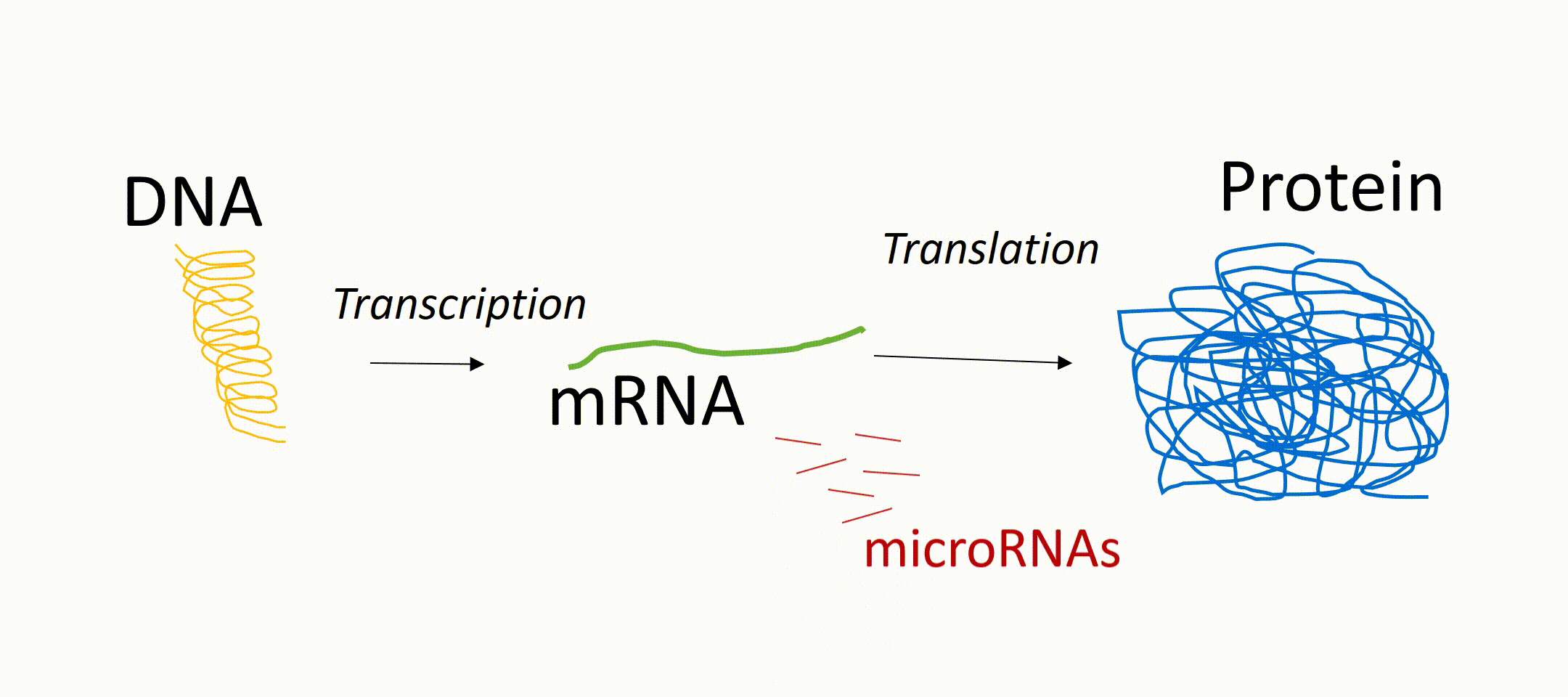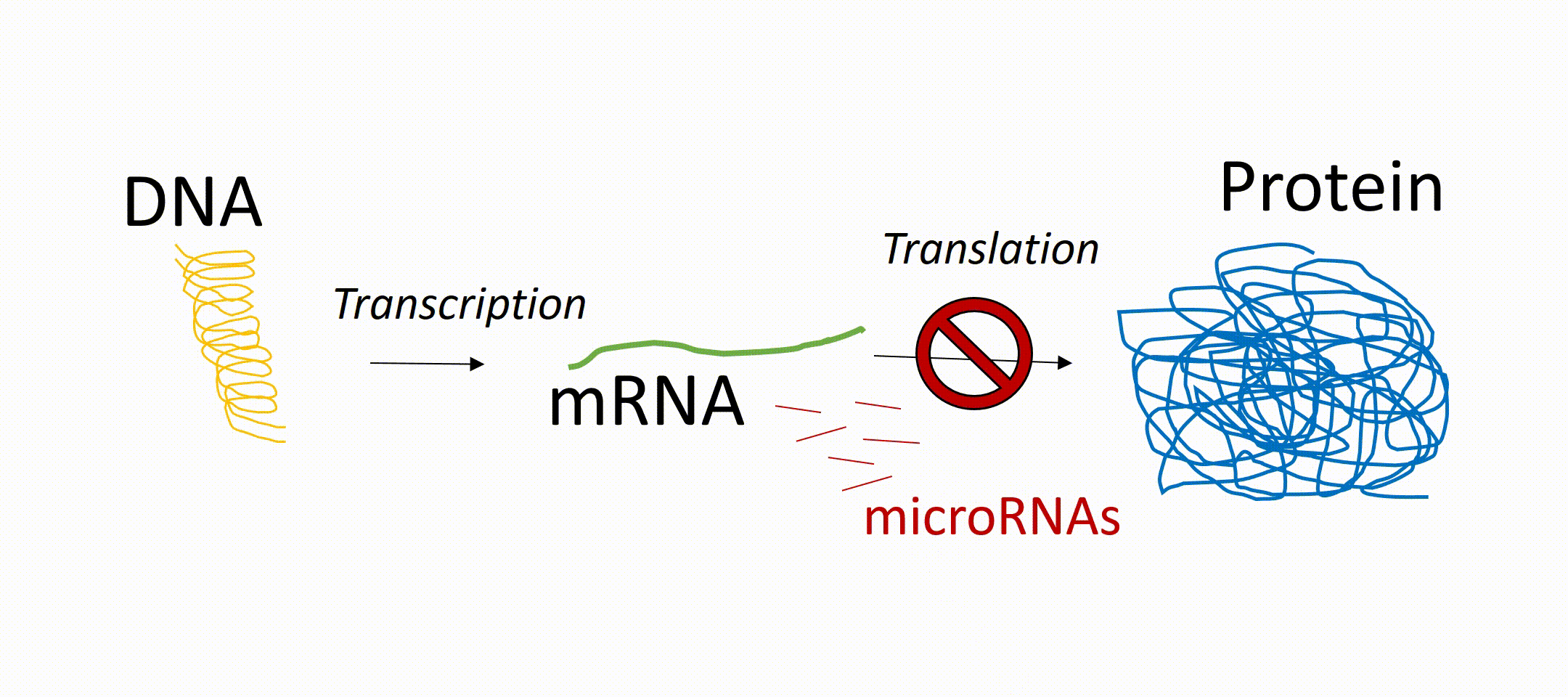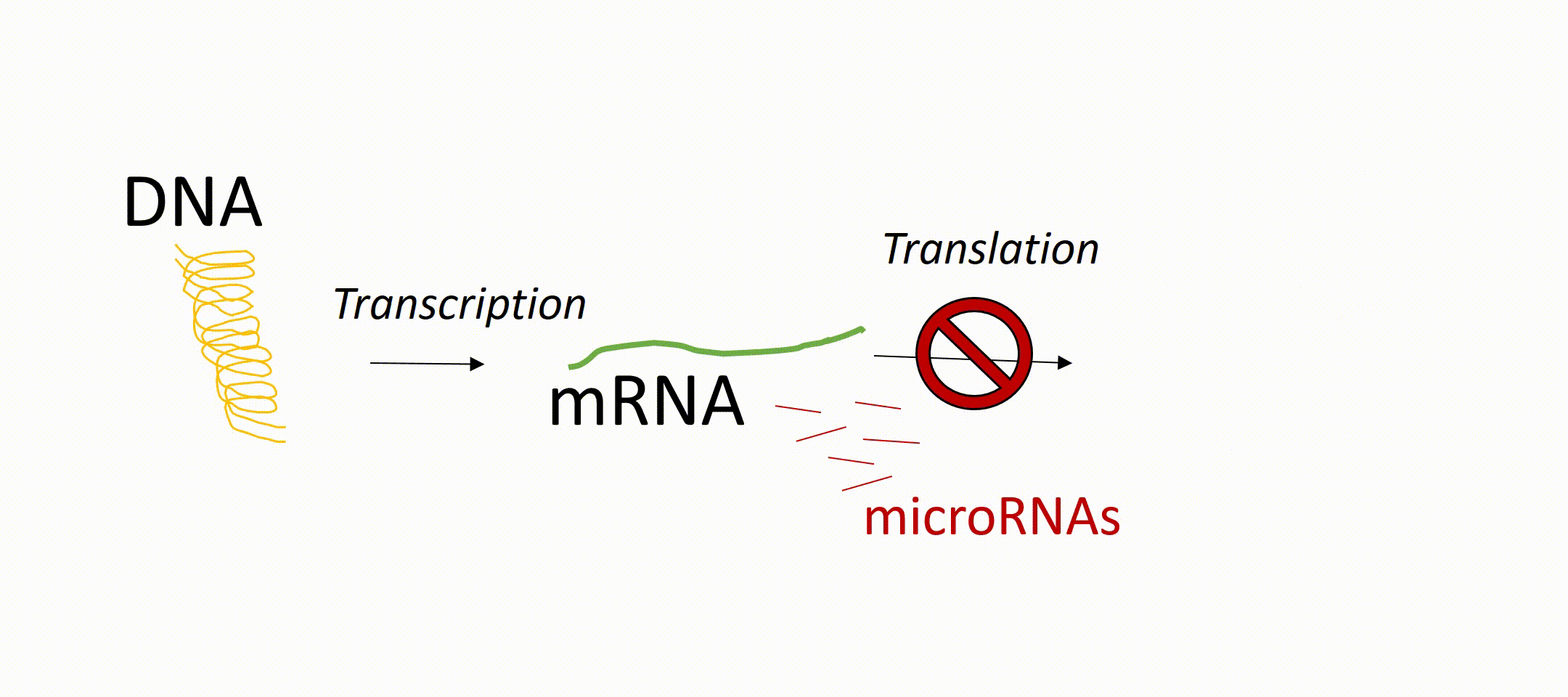
microRNAs or short miRNAs are molecules known to play in role in Müller glia development and function. microRNAs are small RNA molecules present in every cell of the body and act as so-called translational repressors. That means that messenger RNA (mRNA, transcribed from DNA) is not translated into protein. In other words, miRNAs are epigenetic regulators that prevent the generation of proteins even if the gene of this protein is active. These proteins can be receptors on the cell surface or transcription factors inside the nucleus. About 1000 different miRNAs have been identified so far and it is known that they have an impact on development, independent of tissue origin and cell type. However, their expression pattern can vary between different cell types, developmental stages (maturation of a cell), as well as physiological and pathophysiological conditions. For the latter, there is increasing evidence that microRNAs play an important role in various diseases and can be used as a biomarker for certain diseases. miRNAs play also an important role during retinal regeneration in fish by regulating the conversion of the glia into progenitor cells.
Reprogrammed primary mouse Muller glia after miRNA treatment
The Role of microRNAs in Retinal Glia Function
In my laboratory, we investigate
1) The role of miRNAs in Müller glia reprogramming. This includes the role of miRNAs during late retinal development in order to understand if miRNAs are required for the generation of late-born retinal cells, which include rod photoreceptors and bipolar cells (interneurons), as well as Müller glia. miRNAs that are required for cell fate development are very likely successful reprogramming factors.
2) The impact of miRNAs in the different phases of glial activation after injury and/or disease.
This will give us a better understanding of the underlying mechanisms of regeneration and gliosis. The long-term goals are 1) to utilize microRNAs as a tool for regenerative medicine by stimulating endogenous glia to replace lost neurons. And 2) to develop new approaches and therapies to attenuate the glial response after damage to reduce secondary neuronal loss (neuroprotection) and allow potential axonal regeneration of remaining neurons.
Methods and Techniques
Established methods in the lab include:
transgenic mouse models and in vivo manipulations of microRNAs
primary cell cultures (see protocol published with Jove: https://dx.doi.org/10.3791/63651-v)
immortalized cell line cultures (in vitro)
organotypic explant cultures (ex vivo)
microRNA profiling and analysis
bulk and single-cell RNA-sequencing
light damage model
OCT
ERG
protein assays
fluorescence microscopy including time-lapse and confocal laser scanning microscopy
and much more.
Ongoing research based on PREVIOUS work
microRNA sets in Müller glia, neurons, and retinal progenitor cells (RPCs).
1: Müller Glia have a specific set of mircoRNAs
Müller glia have a specific set of microRNAs different from neurons and progenitor cells. However, besides distinct miRNA sets, there are also miRNAs that Müller glia share with other cell types.
Wohl, S. G. and Reh, T.A., 2016. The microRNAs expression profile of mouse Müller glia in vivo and in vitro. Scientific Reports 6, 35423; doi 10.1038/srep35423.
Wohl et al., 2019. MicroRNAs miR-25, let-7 and miR-124 regulate the neurogenic potential of Muller glia in mice. Development 146;10.1242/dev.179556
2: microRNAs can convert Müller glia into retinal Progenitors
The microRNAs neuronal microRNA miR-124 (in combination with miR-9 and miR-9*) can reprogram Müller glia into Ascl1+ retinal progenitor cells that give rise to retinal neurons. The combination of miR-124/9/9* together with the transcription factor Ascl1 accelerates reprogramming. Moreover, over-expression of the retinal progenitor microRNA miR-25 and inhibition of the Müller glia microRNA let-7 leads to conversion of Müller glia into retinal progenitors and subsequent neuronal differentiation.
Wohl, S. G. and Reh, T.A., 2016. miR-124-9-9* potentiates Ascl1-induced reprogramming of cultured Müller glia. Glia 64, 743-762.
Wohl, S. G., Hooper M. and Reh, T.A., 2020. MiroRNAs miR-125, let-7 and mIR-124 regulate the neurogenic potential of Müller glia in mice. Development, 146.
microRNAs convert primary Müller glia into Ascl1 expressing retinal progenitor cells that differentiate into network-forming neurons.
3: microRNAs are essential for Müller glia function and overall retinal health
The depletion of microRNAs in Müller glia (by deleting Dicer, the enzyme that generates mature microRNAs) leads to significant disruptions in the retinal architecture. Müller glia proliferate, migrate and form strange aggregations but do not function properly anymore. As a consequence, photoreceptors die and a phenotype that resembles retinitis pigmentosa can be observed over time. Experiments using organotypic explants cultures show that supplementation with miRNAs helps to rescue the Müller glia phenotype and overall retinal architecture. This implies that dysregulation of miRNAs in Müller glia could be an additional cause of degenerative diseases and consequently, treating diseased retinas with miRNAs could help rescue the tissue.
Wohl, S. G., Jorstad, N. L., Levine, E., and Reh, T.A., 2017: Müller glial microRNAs are required for the maintenance of glial homeostasis and retinal architecture. Nature Communications, 8(1):1603. DOI: 10.1038/s41467-017-01624
4: Reactive Müller glia lose miRNAs
Light damage is a model to induce massive degeneration as it occurs during retinitis pigmentosa or AMD. This injury model leads, similar to other damage paradigms, to reactive gliosis. We found that Müller glia in severely damaged retinas (10,000 lux, 8 h) have significantly reduced levels of miRNAs. This profile resembled the profile we obtained after miRNA depletion (Dicer-cKO mouse). Only a few miRNAs were found to be upregulated after damage. Interestingly, known upregulated generic gliosis genes such as GFAP and Cxcl10 were found to be targeted by Müller glia miRNAs. Moreover, we found an upregulation of stress genes including Maff and Atf3, in both, reactive glia after light damage and mRNA-depleted Müller glia, that were also found to be regulated by Müller glia miRNAs. This suggests that miRNA treatments (supplementation) could attenuate the glial stress response/gliosis that occurs during degenerative retinas.
Kang, S., Larbi, D., Andrade M.D., Reardon, S., Reh, T.A., Wohl, S.G., 2020. A comparative analysis of reactive Müller glia gene expression after light damage and microRNA-depleted Müller glia – focus on microRNAs. Frontiers in Cell and Developmental Biology: 620459 doi: 10.3389/fcell.2020.620459
LAB Members
Email: swohl@sunyopt.edu
phone: +1 212 938 5822
Stefanie G. Wohl, Principal Investigator
I was born in Germany and studied Biology (Diploma) at the Friedrich Schiller University (FSU) of Jena, Germany, and was always fascinated by neurobiology, regeneration, and stem cell research. In 2003, I started working as an undergraduate in the laboratory of Stefan Isenmann, M.D., at the Clinic of Neurology (FSU) in Jena which investigated axonal regeneration of the optic nerve. I continued my work as a graduate student under the supervision of Stefan Isenmann, M.D., Christian Schmeer, Ph.D., and Jürgen Bolz, Ph.D., my professor of neurobiology, who was trained by Heinz Wässle, Ph.D. My research topic was the identification of putative stem cell-like cells after moderate and severe injury. This included studies of neurodegeneration, gliosis, and immunological cell responses. At this time I became intrigued with Müller glia in the retina via the work of Drs. Andreas Reichenbach and Andreas Bringman from the University of Leipzig. By coincidence, I discovered a subtype of microglia that transiently increased in number after optic nerve injury expressing a marker that was primarily associated with stem/ progenitor cells (nestin). In 2011, I received my Ph.D. (neuroscience/ ophthalmology) with the highest honors (summa cum laude) from the Friedrich Schiller University of Jena. For my post-doc training, I decided to go abroad and joined the laboratory of Tom Reh at the University of Washington in Seattle. During this time I discovered my interest in and passion for microRNAs and focused my research on Müller glia. In September 2018, I became an Assistant Professor at the State University of New York, College of Optometry in the Department of Biological and Vision Sciences. My laboratory is the only molecular biology lab in the College and our research focus is on understanding the role of microRNAs in retinal glial function with the overall goal - to fight blindness.
I was a recipient of a Research Fellowship from the German Research Foundation (DFG, 2014-2016) and the SUNY Empire Innovation Grant (2018-2022). Since 2022 my research has been funded by the NIH (R01EY032532).
email: mandrade@sunyopt.edu
phone: +1 212 938 5822
Monica Andrade, Vision Research Coordinator
Monica joined the lab in 2019 as a Senior Research Support Specialist. She was promoted to Vision Research Coordinator in 2021 but is still affiliated with the lab. She received her Bachelor of Arts from Rutgers University in New Brunswick, NJ with Biology as a major. She received a Master of Science from Long Island University, Brooklyn campus, with Medical Microbiology as a specialty.
She worked in multiple projects involving epigenetic studies of the transcriptional repressor Zinc finger protein 57homolog (ZFP57), characterization and biological function of recombinant tissue factor pathway inhibitor beta (TFPIß), and the construction of 16S rRNA libraries for studies of the human microbiome.
email: skang@sunyopt.edu
Seoyoung Kang MMSc, PhD student
Seoyoung received her Bachelor of Optometry in 2015 and is a licensed optician in South Korea. In 2017, she received her Master of Medical Science in from Konyang University, Medical School, South Korea. She is a certified member of the American Board of Opticians (ABO) and National Contact Lens Experts (NCLE) of the State of California and did an internship at Visus Contactlinsen GmbH Hamburg, Germany, in 2013.
Seoyoung has experience with injury models and molecular biology techniques. During her Masters studies (2015-2017), she worked on a project about the role of Wnt signaling on blue-light induced photoreceptor cell damage in the mouse retina. The blue-light light damage is a model for age-related macular degeneration (AMD). In 2018, she worked on a Myopia project in chicken at the Konyang University Hospital and investigated the specific gene expression patters after treatment with different wavelength of visible light.
Seoyoung was accepted as PhD Student in summer 2019 and awarded with the Graduate Assistantship Stipend. She joined the Wohl Lab in July 2020 after successful lab rotation. Her project is about the role of microRNAs in postnatal development with focus on late retinal progenitor cells as well as microRNAs as reprogramming factors.
email: dlarbi@sunyopt.edu
Daniel Larbi OD, PhD Student
Daniel is a qualified optometrist who received his Doctor of Optometry (OD) degree from Kwame Nkrumah University of Science and Technology (KNUST), Ghana. He worked for a year as a teaching and research assistant at the same university. There, he studied myopia (near-sightedness), in particular the correlation between the shape of the eye and myopia progression, and focused on peripheral refraction in high myopes.
Daniel was accepted as PhD student at SUNY Optometry in Summer 2019 and awarded with the Graduate Assistantship stipend. He joined the Wohl Lab in July 2020 after a successful lab rotation, to pursue his passion for research. He has a high interest in understanding molecular mechanisms of injury and disease and his project is about the role of Müller glia microRNAs after retinal injury and in retinal disease.
email: arief@sunyopt.edu
Alexander Rief, OD/MS Student
Alex received his Bachelor of Science (BS) from the University at Albany while being enrolled in the 3+4 Joint Program with the SUNY College of Optometry. After starting the graduate program, he was accepted into the Dual OD/MS Program at the end of 2021. He then joined the Wohl Lab at the start of 2022. Alex has an interest in the field of ocular disease and his research is focused on characterizing Muller cells in a retinal degeneration model replicating retinitis pigmentosa. He has been awarded the T35 Research Grant in the summer of 2022.
email: kbatsuuri@sunyopt.edu
Dr. Khulan Batsuuri, postdoc
Khulan received her medical degree from the Health Science University, School of Medicine in Mongolia. She matriculated into the Residency program of Ophthalmology at the First Central Hospital of Mongolia, where she worked as a resident doctor for over a year, focusing on monitoring patients' glaucoma progression.
Upon arrival in the United States, she joined Dr. Dong Feng Chen's Lab at Schepens Eye Research Institute, Harvard Medical School, as a Research Scholar. She worked on an epigenetic project, studying the role of transcription factors Ezh2 and G9a in retinal ganglion cell survival.
Khulan was accepted as a PhD student at SUNY, College of Optometry, in 2017 and was awarded the Graduate Assistantship Stipend. For her doctoral thesis, she studied the “role of gap junction protein Connexin43 in glaucoma and optic nerve injuries” under the mentorship and guidance of Dr. Miduturu Srinivas and received her PhD in 2023.
Khulan joined the Wohl lab as a post-doctoral Research Scholar in 2024.
email: schen6@sunyopt.edu
Shaoheng (Raven) Chen OD, Residency/MS Student
Raven received his Doctor of Optometry from SUNY in 2024 and is a licensed optometrist in New York. In 2020, he received Bachelor of Medicine from Wenzhou Medical Univerisity, in which he had experience of developing a real-time imaging platform of human oxygen metabolism with visible spatially modulated light.
Raven was accepted in combined Master and Residency program 2024-2026 at SUNY Optometry. He has strong motivation to integrate basic science to clinical Optometry. His project is to apply the technology of Müller glia microRNAs knockout to the real disease mouse model of retinitis pigmentosa.
email: mlimacarneiro@sunyopt.edu
Mariana Lima Carneiro, Intern and Research Technician
Mariana earned her Bachelor’s in Biology from the University of Porto, Portugal, in 2022, followed by a Master’s in Neuroscience from the University of Strasbourg, France, in 2024. She gained diverse research experience through internships at the Abel Salazar Institute of Biomedical Sciences (ICBAS) in Portugal, at the French National Centre for Scientific Research (CNRS), and at the Korea Advanced Institute of Science & Technology (KAIST) in South Korea. Her Master’s thesis at KAIST focused on the roles of YAP/TAZ and PROX1 in regulating Müller glia reprogramming upon injury.
Mariana joined the Wohl Lab as an Intern/Research Technician in 2024 to deepen her exploration of molecular mechanisms in retinal injury and disease. She is particularly interested in the behavior of Müller glia following injury and in microRNAs as reprogramming factors.
Alumni
Konstantin Hahne, Master’s student (Optometry), Berliner Hochschule für Technik
Eik Bruns, Master’s student (Optometry), Berliner Hochschule für Technik, DAAD stipend recipient
T-35 and SUNY SUMMER STIPEND RECIPIENTS
Maxine Humphrey, O.D. cand., SUNY-Optometry (Summer 2024)
Faiz Khan, O.D. cand., SUNY-Optometry (Summer 2024)
Julia Jager O.D. cand., SUNY-Optometry (summer 2023)
Alexander Rief O.D. cand., SUNY-Optometry (summer 2022)
Quynh Nguyen O.D. cand., UC Berkeley School of Optometry (summer 2019)








Join us!
Are you interested in becoming part of our team? We are currently seeking a highly motivated team member with a strong molecular biology/genetics background. Interns/trainees with interest in cellular and molecular vision science are also very welcome to join our lab!
For more info about the PhD student programs see https://www.sunyopt.edu/education/admissions/graduate_programs
For general inquiries, please use the contact form below. For an application, please sent your letter and detailed CV to swohl@sunyopt.edu.
location
The State University of New York
College of Optometry
Department of Biological and Vision Sciences
33W 42nd Street 10036, New York, NY
Email: swohl@sunyopt.edu
Phone office: +1 212 938 4069
Phone lab: +1 212 938 5822
Publications
Holland, A. M., Jeohoul, R., Vranken, J., Wohl, S.G., Boesmans, W., 2025. microRNA regulation of the enteric nervous system development and disease. Trend in Neuroscience. https://doi.org/10.1016/j.tins.2025.02.004.
Batsuuri, K., Toychiev, A., Viswanathan, S., Wohl, S.G., and Miduturu, S., 2025. Targeting Connexin 43 in Retinal astrocytes Promotes Neuronal Survival in Glaucomatous injury. GLIA.
Books
“Neuroscience and Biobehavioral Psychology” Chapter “Müller glia Development” Wohl S.G., Editor in Chief: Patricia d’Amore, Section Editor: Nadean Brown. Section Title: Ocular Development. https://doi.org/10.1016/B978-0-443-13820-1.00126-2
"Molecular Therapies for Inherited Retinal Diseases" Chapter “Retinal miRNA functions in health and disease.” Zuzic M., Rojo Arias J. E., Wohl S.G., and Busskamp V., reprint. https://doi.org/10.3390/books978-3-03943-177-9, ISBN 978-3-03943-176-2 (Hbk); ISBN 978-3-03943-177-9 (PDF), published: October 2020